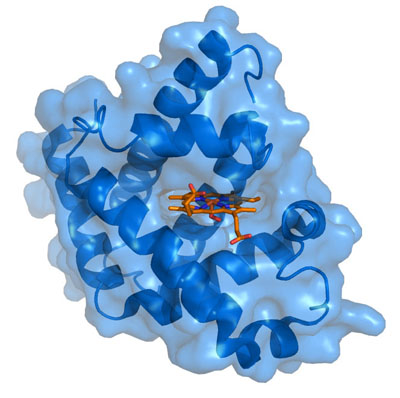
 ingredient is a single charged iron atom (Fe). While hemoglobin, found in red blood cells, transports oxygen from the lungs to all the tissues of the body, myoglobin functions to store excess oxygen within the muscle cell, to help buffer supply during periods of excessive demand. It also transports oxygen from the cell wall to the mitochondria. Even with help from the myoglobin, it's often the case that muscles have to resort to anaerobic metabolism under strenuous exercise, during which lactic acid is built up and released into the blood stream.
ingredient is a single charged iron atom (Fe). While hemoglobin, found in red blood cells, transports oxygen from the lungs to all the tissues of the body, myoglobin functions to store excess oxygen within the muscle cell, to help buffer supply during periods of excessive demand. It also transports oxygen from the cell wall to the mitochondria. Even with help from the myoglobin, it's often the case that muscles have to resort to anaerobic metabolism under strenuous exercise, during which lactic acid is built up and released into the blood stream. Myoglobin exists in at least three distinct forms, which can be characterized as Mg+2 (Ferrous), Mg+3 (Ferric), and Mg+4 (Ferryl), depending upon the amount of charge that is present on the central Iron atom. As Mg+2, its healthy state, it will readily take up oxygen and store it, whereas, when converted to Mg+3 by the addition of a proton, it becomes inert. However, with the addition of yet another proton, it becomes Mg+4, a highly toxic reactive agent that will begin to break down the fatty acids contained in the outer cell wall of the muscle cell (so-called peroxidative damage)[35], and go on to destroy the cholesterol in the cell wall as well [31]. Myoglobin becomes Ferryl myoglobin in the presence of excess amounts of free radicals, i.e., under oxidative stress induced by highly reactive oxygen compounds like hydrogen peroxide. Recall that, with statin therapy, hydrogen peroxide is generated in the mitochondria because the process of breaking down oxygen and converting it to water is incomplete -- due to the insufficient supply of coenzyme Q10.
An excellent article describing the process by which a cell is injured by oxidative stress was written by John Farber in 1994 [13]. He wrote: "All aerobic cells generate, enzymatically or nonenzymatically, a constitutive flux of O2-, H2O2, and possibly *OH. At the same time, the abundant antioxidant defenses of most cells, again both enzymatic and nonenzymatic, prevent these species from causing cell injury. Nevertheless, there are situations in which the rate of formation of partially reduced oxygen species is increased and/or the antioxidant defenses of the cells are weakened. In either case, oxidative cell injury may result." [14, p. 17]. The process of aerobic oxidation of food sources to generate energy is confined to the mitochondria in order to protect the constituents in the cytoplasm as much as possible. But myoglobin is tasked with transporting oxygen from the cell wall through the cytoplasm to the mitochondria. It can't avoid oxygen exposure, and when it delivers the oxygen, it necessarily has to come in contact with these toxic intermediate products of the process that ultimately converts oxygen to water. One of the most important roles of coenzyme Q10 in the muscle cells is to neutralize the damage to myoglobin caused by these oxidative agents.
When a person suffers from a heart attack (ischemic event), their muscles experience an extreme lack of oxygen due to the heart's temporary inability to pump blood. However, one of the most dangerous aspects of a heart attack is the so-called reperfusion period, when blood circulation is restored, but after the cells have suffered injury as a consequence of oxygen deprivation [29]. This condition is especially problematic for the heart muscle, since it is so crucial to survival. In a study involving rats that had suffered from heart attacks, it was proposed that the injury is a direct consequence of exposure to the Fe+4 form of myoglobin (ferryl myoglobin) [1]. Because the cells have been unable to maintain their physiological state during the deprivation period, they are highly vulnerable to oxidative stress.
Once the fatty acids in a muscle's cell wall are broken down due to exposure to toxic Ferryl myoglobin, the cell rapidly disintegrates. Because the cell wall is no longer impermeable to ions, large amounts of calcium start rushing into the cell, and soon after it dies [14]. The debris of the dead and dying cells gets dispersed into the blood stream, and makes its way to the kidneys for disposal. This causes a tremendous load on the kidneys which can sometimes lead to their failure as well [47], and the situation cascades into a downward spiral.
In 1994, Mordente et al. published a paper that investigated in vitro the degree to which coenzyme Q could protect myoglobin from oxidative damage [28]. Their results showed convincingly that coenzyme Q can work as a natural antioxidant for myoglobin. To quote the last sentence of their abstract: "Collectively, these studies suggest that the proposed function of coenzyme Q as a naturally occurring antioxidant might well relate to its ability of reducing H2O2 [hydrogen peroxide]-activated myoglobin. Coenzyme Q should therefore mitigate cardiac or muscular dysfunctions that are caused by an abnormal generation of H2O2."
4 comments:
Check out all the Healthcare programs available in fields like Dentistry, Nursing, Pharmacology, and more from HealthcareColleges.net! You can find campus and online schools, full on degree programs, or just quick technical certification training courses. If you’re looking to go to a Healthcare School then you need to check them out!
This is perfect I've heard that I can get different supplements of this protein combined with Viagra Vs Cialis, I've heard that is for people who lift weight, I'd like to know if it really works.
So is it good for you?
Nice article. Thanks for sharing this post with us. Know about the best diagnostic medical center in Kerala.
Post a Comment