Whenever oxygen is in short supply, or if the mitochondria are dysfunctional, the cell has alternative ways to generate energy (e.g., fermentation), which take place, in the absence of oxygen, in the main compartment of the cell, called the cytoplasm. These processes require exchanges of nutrients between the muscles and the liver, and they require the assistance of special enzymes which then show up in the blood stream. These are the very same enzymes whose concentrations are monitored to detect whether a statin drug may have damaged the muscles.
If you don't feel compelled to know the details of how all these processes work, you could skip this section and section 5, and, I think, still be able to follow the rest of the story.
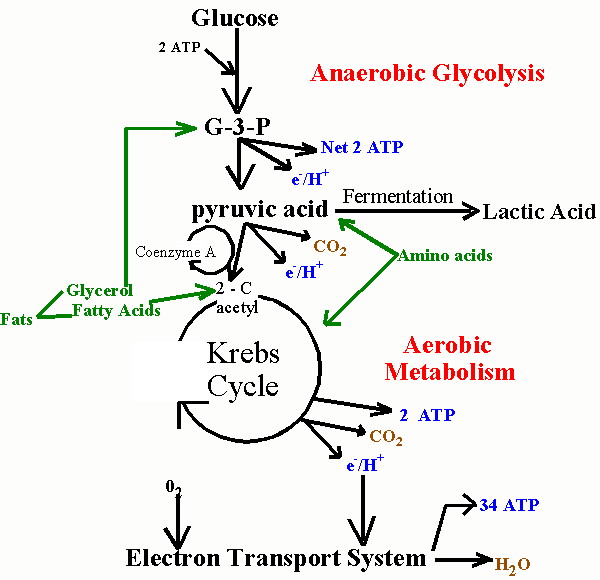 In order to explain how statins damage muscles, I will first need to explain how muscles manage their energy needs. Muscles require a significant amount of energy to contract, and they get most of this energy by breaking down fatty acids and glucose obtained originally from food sources. Like all eukaryotic cells (cells containing a nucleus), muscle cells are able to generate a lot of energy through aerobic (oxygen-requiring) processes that are sequestered within special energy-generating subregions of the cell called mitochondria. This aerobic metabolic process is highly efficient, generating as many as 30 units of ATP (Adenosine Triphosphate) for each molecule of glucose. ATP can be thought of as an energy currency, because it can be easily broken down to AMP (adenosine monophosphate), releasing the stored energy in the process, which will then fuel cell contraction.
In order to explain how statins damage muscles, I will first need to explain how muscles manage their energy needs. Muscles require a significant amount of energy to contract, and they get most of this energy by breaking down fatty acids and glucose obtained originally from food sources. Like all eukaryotic cells (cells containing a nucleus), muscle cells are able to generate a lot of energy through aerobic (oxygen-requiring) processes that are sequestered within special energy-generating subregions of the cell called mitochondria. This aerobic metabolic process is highly efficient, generating as many as 30 units of ATP (Adenosine Triphosphate) for each molecule of glucose. ATP can be thought of as an energy currency, because it can be easily broken down to AMP (adenosine monophosphate), releasing the stored energy in the process, which will then fuel cell contraction.Unfortunately, the process of metabolizing food sources for energy is quite complex. I have found two images which depict food metabolism in complementary ways, where one (above, right) shows chemical reactions and the other (below, left) schematizes the regions of the cell that are involved. They use slightly different nomenclature, but I will try to link them together when necessary. When glucose first enters the cell (mediated through insulin), it is converted to pyruvate (also called pyruvic acid) in the cell's cytoplasm (the main compartment of the cell). This process releases a small amount of ATP, but does not require oxygen, which makes it useful when oxygen is in short supply. The pyruvate can also be broken down to lactate (also called lactic acid) (fermentation, oxygen absent in the figure below) in the cytoplasm, without requiring any oxygen, so-called anaerobic metabolism, to release additional energy. This pathway is important to muscle cells under conditions of extreme exercise, when oxygen supplies become depleted.
To generate a much larger amount of ATP requires the help of the mitochondria (the large oval purple-shaped object in the figure), and involves a well known process referred to multiple ways: as the respiratory or electron transport chain, the Tricarboxylic acid (TCA) cycle or the Krebs Cycle (TCA cycle, oxygen present in the figure below; Krebs Cycle, Aerobic Metabolism in the figure above). The process is tricky, because oxygen molecules (O2) have to be split apart, and, during intermediate stages, dangerous free radicals are lying around (individual negatively charged oxygen atoms that have not yet fully combined with hydrogen (H+) to form the very stable molecule, water (H2O)).
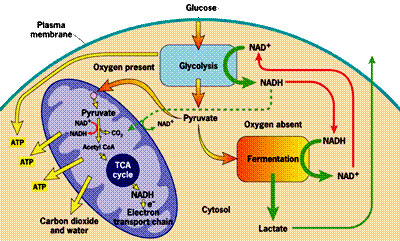 These free radicals are highly reactive. Antioxidants are compounds that can absorb these free radicals and render them harmless. Two very important antioxidants that play a critical role in the electron transport chain are coenzyme Q10 (also known as ubiquinone) and cytochrome c.
These free radicals are highly reactive. Antioxidants are compounds that can absorb these free radicals and render them harmless. Two very important antioxidants that play a critical role in the electron transport chain are coenzyme Q10 (also known as ubiquinone) and cytochrome c. 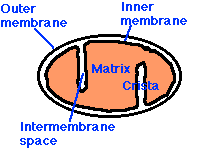 The small figure to the left shows a schematic of a mitochondrion, and the larger figure below shows a more detailed explanation of the electron transport chain process that takes place along the wall enclosing the mitochondrion, generating a large percentage of the cell's energy needs in the process. The electron transport chain injects protons (H+) into the intermembrane space, essentially creating a battery (charge differential across the membrane) that can then complete the process of converting (spent) AMP back to ATP as a renewed energy source. If there is an insufficient supply of coenzyme Q10 (also known as "ubiquinone", the "Q" in the figure), then the
The small figure to the left shows a schematic of a mitochondrion, and the larger figure below shows a more detailed explanation of the electron transport chain process that takes place along the wall enclosing the mitochondrion, generating a large percentage of the cell's energy needs in the process. The electron transport chain injects protons (H+) into the intermembrane space, essentially creating a battery (charge differential across the membrane) that can then complete the process of converting (spent) AMP back to ATP as a renewed energy source. If there is an insufficient supply of coenzyme Q10 (also known as "ubiquinone", the "Q" in the figure), then the 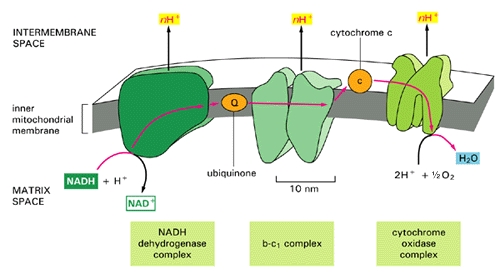 electron transport chain will not work as efficiently. Hydrogen ions will leak back into the mitochondrion through a passive process, requiring a much larger expenditure of energy to push them back out [20]. The battery charge will be reduced, and there will be a decrease in the amount of ATP that can be generated. The net effect will be very similar to the effect of insufficient oxygen, with respect to energy generated. However, it will be much more damaging because, instead of being absent, the oxygen is present but is only partially converted to water (2H+ + 1/2 O2 -> H2O at the right of the figure), since the chain of events is held up at the "Q" position. Various highly toxic charged ions containing oxygen, such as -OH, H2O2 (hydrogen peroxide) and *OH, will linger and wreak havoc on the muscle cell, as you will see later.
electron transport chain will not work as efficiently. Hydrogen ions will leak back into the mitochondrion through a passive process, requiring a much larger expenditure of energy to push them back out [20]. The battery charge will be reduced, and there will be a decrease in the amount of ATP that can be generated. The net effect will be very similar to the effect of insufficient oxygen, with respect to energy generated. However, it will be much more damaging because, instead of being absent, the oxygen is present but is only partially converted to water (2H+ + 1/2 O2 -> H2O at the right of the figure), since the chain of events is held up at the "Q" position. Various highly toxic charged ions containing oxygen, such as -OH, H2O2 (hydrogen peroxide) and *OH, will linger and wreak havoc on the muscle cell, as you will see later. There are a number of rare genetic disorders that involve mutations in genes encoding enzymes that operate within the electron transport chain [23][32]. Particularly relevant to our story are Complex I enzymes, because Coenzyme Q10 is one of them. An interesting case study involved two sisters [23], both of whom suffered from a genetic mutation leading to a defect identified to be associated with the NADH-Coenzyme Q10 complex. As would be predicted, they suffered from substantially decreased rates of respiratory metabolism (the process discussed above). They also were extremely weak and had marked intolerance to exercise. When they exercised, their levels of lactate and pyruvate rose sharply in the blood, an indication that they were relying on anaerobic fermentation in the cytoplasm rather than aerobic metabolism in the mitochondria to meet their energy needs.
3 comments:
The majority of website designers focus wrongly on designing websites that appeal to their clients which leads to websites that are poorly designed to serve the needs of the visitor or the client. I recently concluded the majority of today's websites are no better designed now than 5 years ago. Testosterone booster reviews
Thank you so much for such a valuable content. Great information. Know about the best medical scanning lab in Kerala.
Informative article. Thanks for sharing this valuable post with us. Get to know about the best medical oncologist in Kerala.
Post a Comment